Introduction: There is limited data comparing infectious toxicities in patients with relapsed/refractory multiple myeloma (RRMM) treated with T-cell engaging therapies, which include chimeric antigen receptor T-cell therapy (CAR-T) and bispecific antibodies (BsAb). There are now three US FDA approved B-cell maturation antigen (BCMA)-targeting T-cell engagers, and several more in ongoing clinical development. Awareness regarding their infectious toxicities may inform treatment selection and mitigation strategies.
Methods: We conducted a single-center, observational study in patients with RRMM comparing infectious complications in patients treated with a commercial or investigational autologous BCMA-targeting CAR-T versus patients treated with a commercial or investigational BCMA-targeting BsAb. The date of CAR infusion (day 0) was between 03/22/2017 - 02/27/2023 for the CAR-T patients. For the BsAb cohort, the date of treatment initiation (day 0) was between 01/21/2020 - 02/07/2023. All infection-specific variables were collected from day 0 until the date of next line of therapy or last follow-up, with a data cut-off of 06/01/23. Infectious events were graded according to CTCAE version 5.0. Prophylactic antimicrobials were administered according to institutional and protocol guidelines. The primary endpoint was the incidence of severe (grade ≥3) infections. Secondary objectives included the time to 1 st infection, infection rate over time, infectious organisms, the impact of prolonged cytopenias, and the utility of intravenous immunoglobulin (IVIg) administration in preventing infections.
Results: Of the total 147 patients, there were 92 CAR-T and 55 BsAb treated patients. The median age of the CAR-T and BsAb cohort was 62yrs vs 65yrs, respectively (P=0.043). CAR-T patients had a median of 6.5 prior lines of therapy (IQR 5-8) versus 6.0 (IQR 4-9) in the BsAb cohort (P = 0.7); 97% of CAR-T patients had a prior autologous transplant compared to 75% in the BsAb cohort (P < 0.001). In keeping with current clinical practice, 18 patients (33%) in the BsAb cohort had prior exposure to CAR-T whilst no patients in the CAR-T cohort had prior BsAb exposure. The median follow-up duration for infectious events was similar in both groups at 5.8 months (IQR 3.8-9.2) for CAR-T and 4.3 months (IQR 3.2-9.8) for the BsAb cohort.
A total of 209 infections were reported: 115 with CAR-T and 94 with BsAb. In the CAR-T cohort 24/92 patients (26%) experienced ≥1 severe infection, all of which were grade 3 and there were no grade 4 or 5 events. A numerically higher incidence was seen in the BsAb cohort with 21/55 BsAb patients (38%) experiencing ≥1 severe infection (P = 0.14). Nineteen BsAb patients (35%) experienced grade 3 infections, 2 (3.6%) had grade 4 infections and 4 (7.3%) had grade 5 infections. Six CAR-T (6.5%) and 10 BsAb patients (18.2%) had >1 grade 3 infection. The incidence of ≥1 severe infection remained high in the BsAb cohort at 16/37 (43%) even after excluding the 18 BsAb patients with prior CAR-T exposure.
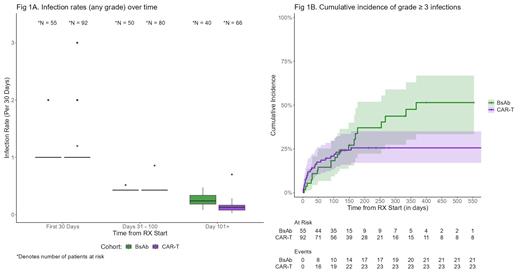
The median time from treatment initiation to the first infection of any-grade was 2.5 months (95% CI 1.2 - NR) with CAR-T compared to 3.4 months (95% CI 1.6 - 6.8) with BsAb (P = 0.6). The rate of any-grade infections was similar between the two groups early post-therapy (before day 100). However, there was a significantly higher infection rate of any-grade after day 100 with BsAb compared to CAR-T with a median of 0.24 (IQR 0.18 -0 .34) vs 0.13 (IQR 0.08 - 0.17) infections per 30 days, respectively (P < 0.001) (Figure 1A). Regarding the time to the first grade ≥3 infection, 79% occurred within day 100 for CAR-T patients compared to only 48% in the BsAb cohort (Figure 1B).
The proportion of bacterial, viral, fungal, and parasitic infections in the CAR-T group was 49% (n = 56), 48% (n = 55), 4.3% (n = 5) and 0.9% (n = 1) respectively, and in the BsAb group was 51% (n = 48), 44% (n = 41), 3.2% (n = 3), and 0 respectively.
Conclusion: Infectious complications were common early after BCMA-targeting BsAb and BCMA CAR-T and declined over time. In this real-world comparison, distinct from their CAR-T counterparts, BsAb recipients appeared to have a more persistent infection risk and higher incidence of severe infections, which included four patients having a grade 5 infection. Further analysis, including the impact of prolonged cytopenias and the utility of IVIg administration will be presented at the meeting.
Disclosures
Shekarkhand:Genentech: Consultancy. Nishimura:Chugai pharmaceutical company: Consultancy; Ono pharmaceutical company: Honoraria. Landau:Alexion Pharmaceuticals, Takeda, Janssen, Prothena, Protego: Research Funding; Karyopharm, Pfizer, Juno, Prothena, Caelum Biosiences, Legend Biotech, Takeda, Janssen, Nexcella: Honoraria. Lahoud:MorphoSys Inc, Kite: Consultancy. Scordo:CancertNetwork (Intellisphere LLC): Honoraria; Angiocrine Bioscience, Inc.: Research Funding; Omeros Corporation: Consultancy, Research Funding; Amgen, Inc.: Research Funding; Medscape, LLC: Honoraria. Shah:BMS: Research Funding; ArcellX: Other: DSMB; Beyond Spring: Research Funding; Amgen: Research Funding; Janssen: Research Funding. Hassoun:Celgene, Takeda, and Janssen Pharmaceuticals: Research Funding. Korde:Amgen, Janssen, Epizyme, AbbVie: Research Funding; Janssen: Other: Advisory Board; CCO, OncLive, Intellisphere, Remedy Health: Consultancy. Shah:C4 Therapeutics: Research Funding; Plantable: Research Funding; M and M Labs: Research Funding; Janssen: Consultancy, Other: Advisory Board, Research Funding; Sanofi: Other: Advisory Board; Bristol Myers Squibb: Consultancy, Other: Advisory Board, Research Funding; Sabinsa: Research Funding. Tan:Takeda: Research Funding; Janssen: Research Funding. Hultcrantz:Amgen, Daiichi Sankyo, GlaxoSmithKline: Research Funding; Curio Science LLC, Intellisphere, Bristol Myer Squibb, GlaxoSmithKline: Honoraria. Giralt:Amgen, Actinuum, Celgene/BMS, Kite Pharma, Janssen, Jazz Pharmaceuticals, Johnson & Johnson, Novartis, Spectrum Pharma, Takeda: Membership on an entity's Board of Directors or advisory committees; Amgen, Actinuum, Celgene/BMS, Omeros, Johnson & Johnson, Miltenyi, Takeda: Research Funding. Usmani:Genentech: Membership on an entity's Board of Directors or advisory committees; K36 Therapeutics: Membership on an entity's Board of Directors or advisory committees; Moderna: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees, Research Funding; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees, Research Funding; TeneoBio: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; SkylineDX: Membership on an entity's Board of Directors or advisory committees, Research Funding; SecuraBio: Membership on an entity's Board of Directors or advisory committees; GSK: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead Sciences: Membership on an entity's Board of Directors or advisory committees, Research Funding; EdoPharma: Membership on an entity's Board of Directors or advisory committees; Array Biopharma: Research Funding; Merck: Research Funding; Pharmacyclics: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol Meyer Squibb: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Membership on an entity's Board of Directors or advisory committees, Research Funding. Mailankody:Caribou Therapeutics: Research Funding; Optum Oncology: Consultancy; Janssen Oncology: Consultancy; Legend Biotech: Consultancy; Physician Education Resource: Honoraria; Takeda Oncology: Research Funding; Janssen Oncology: Research Funding; Fate Therapeutics: Research Funding; Allogene Therapeutics: Research Funding; Bristol Myers Squibb: Research Funding; OncLive: Honoraria; MJH Life Sciences: Honoraria. Lesokhin:Genentech: Research Funding; Iteos: Consultancy; Janssen: Research Funding; BMS: Research Funding; Serametrix (now Caprion): Patents & Royalties; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Arcellx: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal